Early Product Management
Unlocking the Secrets of Pharmaceutical Validation: A Comprehensive Training Guide
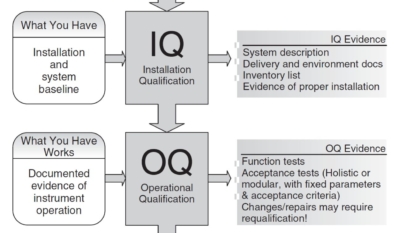
Discover the critical stages of pharmaceutical validation: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Dive into our comprehensive guide that serves as a full training session on ensuring compliance and performance in the life sciences field.
Read More »What happens at the design stage
The design stage covers the period from receiving budget approval to just before embarking on fabrication. Formulate the basic design, based on the equipment specification, and draw up the implementation budget. At this point, an equipment FMEA (see Figure “The Anatomy of an MP Information Utilization System” in lesson 3.5)…
Read More »Making use of information on problems arising during Early Product Management
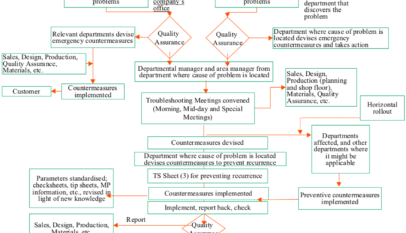
Despite all efforts made to build in quality and factory-friendliness at the design stage, problems may well arise, inside or outside the company, from any point from prototyping through volume production. When this happens, it is important to establish the facts, investigate the causes, send prompt feedback to the preceding…
Read More »Building in quality through control of first-of-run products
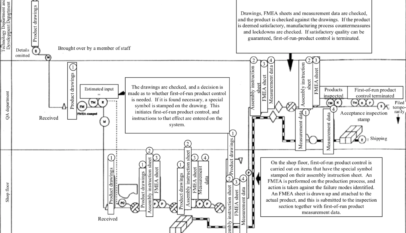
The system ensures that quality is built into the product by making it impossible for any product to be shipped until the production department has carried out a process FMEA, implemented and checked countermeasures, and locked them down.
Read More »