Introduction:
Welcome to the world of pharmaceutical validation, a critical arena where precision meets compliance to ensure the safety and efficacy of medicinal products. In this full training session, we will navigate through the core stages of validation: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each stage represents a cornerstone in the foundation of a robust quality management system.
Design Qualification (DQ): The Blueprint of Success DQ is the starting block where the race toward impeccable quality begins. It focuses on collecting and organizing design requirements and specifications. This phase is all about ensuring that the design of new equipment or systems aligns perfectly with the intended purpose. Evidence in this stage is crucial, comprising of:
- Requirements specifications
- Test evidence
- Quality system/supplier audit
Here, we establish the ‘what’ of our process – what we need the system to do, and what standards it must meet.
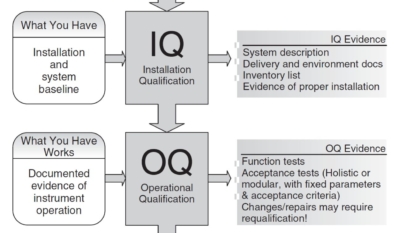
Installation Qualification (IQ): Setting the Stage for Excellence Once the design is locked in, IQ takes the spotlight. It’s where ‘what you have’ is meticulously compared against ‘what you need.’ The installation process is validated to ensure that everything is received as designed and installed correctly. The IQ evidence you collect will include:
- System description
- Delivery and environment documentation
- Inventory list
- Evidence of proper installation
The IQ is your assurance that the system is not only there but also correctly set up, forming a solid baseline for the operations to follow.
Operational Qualification (OQ): The Performance Rehearsal OQ is where we test if ‘what you have’ works as expected under multiple scenarios. This stage is the trial by fire for the equipment, involving:
- Function tests
- Acceptance tests (holistic or modular with fixed parameters and acceptance criteria)
- Documentation of any changes or repairs that may necessitate requalification
It’s a rigorous check to prove that the system operates reliably within the defined parameters.
Performance Qualification (PQ): The Final Act Finally, PQ is the validation of ‘what you have continues to work’ over time. This is not a one-time event but an ongoing commitment. PQ evidence encompasses:
- Verification based on typical application use
- On-site performance
- Periodic review to ensure continuous compliance
It is the ongoing system performance monitoring, ensuring it consistently produces the correct outputs.
To ensure that pharmaceutical products are developed, manufactured, and controlled according to quality standards, companies need to understand and implement DQ, IQ, OQ, and PQ. The purpose of this complete training session was to equip you with the knowledge to confidently navigate the validation process. Every step you take is an important measure towards safeguarding public health.
As we wrap up this session, remember that validation is not just about ticking boxes – it’s about upholding patients’ trust in the treatments they receive. So, let’s commit to excellence and integrity in every phase of pharmaceutical validation.