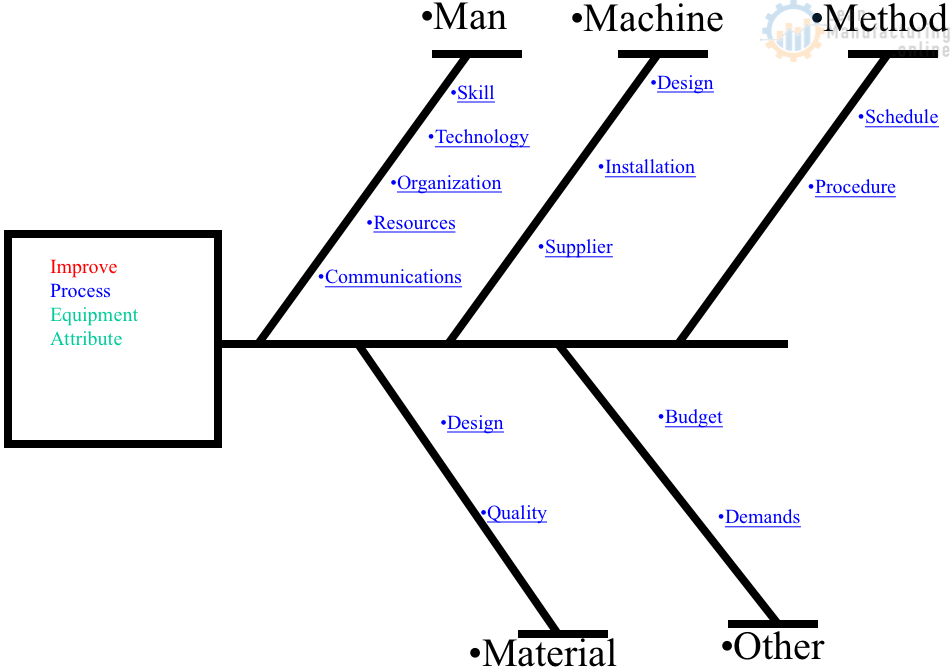
Every workplace undergoes changes, and how we manage them can significantly impact our operations’ success. The 4M changes—Man, Machine, Material, and Method—are critical areas where alterations might occur. Today, we dive into a detailed work instruction (WI) sheet designed to navigate these changes effectively.
Man
Changes in personnel, whether due to leaves, new operators, or job rotations, require a structured response. For instance, when an operator is on leave, a skilled substitute is deployed, and certain checks such as 100% visual inspection and part dimensionality according to the sampling plan are necessary. Training, as per operation standard, is provided to new operators to ensure continuity and quality.
Machine
Machine updates, preventive maintenance, and breakdowns also demand attention. Whether it’s working with a new machine or managing one under repair, the key is to maintain production capacity and quality. After a machine restart due to a power failure, a set-up approval is essential before resuming operations.
Material
The source and grade of materials used in production are pivotal. If the material is received from an unapproved source or a different grade is used, it’s crucial to halt production until set-up approval from quality and the customer is obtained. This ensures compliance and maintains trust.
Method
Any changes in process parameters or methods, like a deviation from the standard process or a new sequence, require a planned approach. Starting production post-approval and ensuring machine parameters are within specifications are part of the standard procedure.
Addressing the Changes
Each change is classified as planned or unplanned, and corresponding actions are taken. For planned changes, pre-approval is often required, whereas unplanned changes necessitate retrospective inspections and containment actions. The focus is on maintaining quality and minimizing disruptions.
Set-Up Approval and Inspection
A recurring theme in the WI sheet is the necessity for set-up approval and thorough inspections. Whether it’s a planned change or an abnormal situation, ensuring all parts are inspected visually and dimensionally as per the sampling plan is mandatory. This vigilance safeguards the production process and upholds standards.
Documentation and Record Keeping
Each step taken in response to 4M changes is meticulously documented. From approvals to inspections and any deviations, keeping a detailed record is paramount for traceability and quality assurance.
Conclusion
The WI sheet for 4M changes is a testament to the robust processes that underpin effective operational management. By adhering to these structured instructions, organizations can navigate changes with confidence, ensuring minimal impact on production and quality.