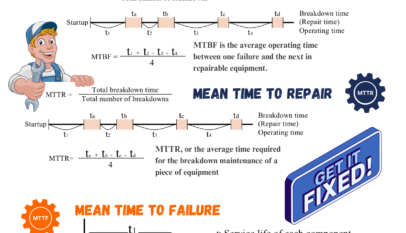
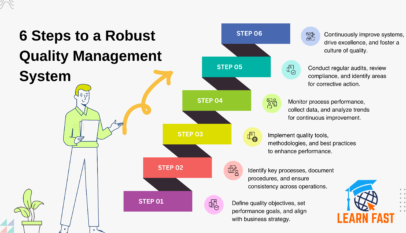
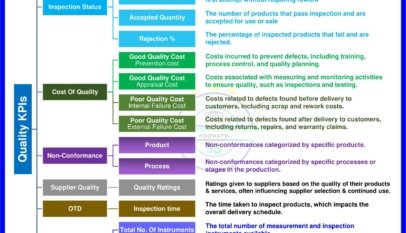
Good Manufacturing Practice (GMP) stands as the bastion ensuring products meet the highest quality standards in the world of pharmaceuticals and health. But what exactly does GMP entail? And how does it permeate every facet of a pharmaceutical company? Let’s take a closer look.
1. Quality Management: The Heart of GMP
Quality isn’t just about the end product; it’s a continuous journey. At the core of GMP is the rigorous commitment to quality management. This encompasses:
- Quality Assurance: The systematic approach to ensure products meet the desired quality criteria.
- Quality Control: The active scrutiny, via regular inspections and evaluations, to ensure consistent product quality.
- Product Quality Reviews: Regular assessments of the product against its intended quality.
- Quality Risk Management: A proactive approach to identifying and managing potential risks that could compromise quality.
2. Personnel: The Drivers of Quality
Quality management isn’t effective without the right team. Ensuring compliance with GMP starts with robust personnel management. Key elements include:
- Meticulous onboarding and offboarding processes.
- Clear and comprehensive job descriptions.
- Continuous training and education.
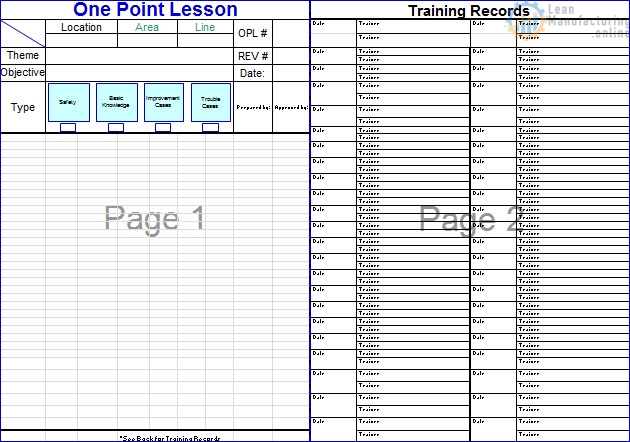
- Maintenance of comprehensive training records for every individual.
3. Documentation: The Backbone of Traceability
Documentation in GMP isn’t just paperwork—it’s a lifeline. It offers a clear audit trail, minimizes errors, and establishes standard procedures. Important documents include specifications, batch records, SOPs (Standard Operating Procedures), and various logs and registers.
4. Premises and Equipment: The Setting for Excellence
The design and layout of manufacturing premises and equipment are pivotal in GMP compliance. Facilities must:
- Adhere to GMP regulatory requirements.
- Facilitate thorough cleaning and maintenance.
- Prevent cross-contamination and dust build-up.
- Offer protection against pests.
5. Operations: Precision in Every Step
In line with GMP, each step of pharmaceutical production requires meticulous attention. Employees must handle materials detailed in the organization’s procedural documents, ensuring consistency and quality at every turn.
6. Incidents: Handling the Unexpected
Despite best efforts, incidents can occur. GMP emphasizes the importance of robust procedures for managing incidents, whether customer complaints, recalls, or counterfeits.
7. Inspections: The Mirror of Compliance
Inspections play a dual role. They ensure GMP compliance and help organizations refine their procedures. These inspections include internal audits as well as regulatory and customer inspections.
8. Contracted Services: Outsourcing with Confidence
GMP extends beyond the immediate organization. External service providers must undergo rigorous audits, from raw material suppliers to pest control agencies. Technical quality agreements are established, and there’s an intricate mapping of the entire supply chain.
Good Manufacturing Practice isn’t a single action but a symphony of intertwined procedures, guidelines, and standards. By prioritizing quality management, investing in personnel, maintaining impeccable documentation, and ensuring excellence in operations and equipment, GMP ensures that the pharmaceutical products reaching consumers are of the highest calibre. It’s a commitment to health, safety, and unwavering quality.