Here, we identify any lapses in the 4-M conditions for each process as found in Step 3 and list them in a Deficiencies Chart. We then work out how to correct these deficiencies (see Table “Chart of Countermeasures for Deficiencies in the 4 Ms”). If the actions required to correct a particular deficiency are immediately obvious, then someone can be assigned to do them right away. If not, however, we go to Step 5 and work out how to proceed.
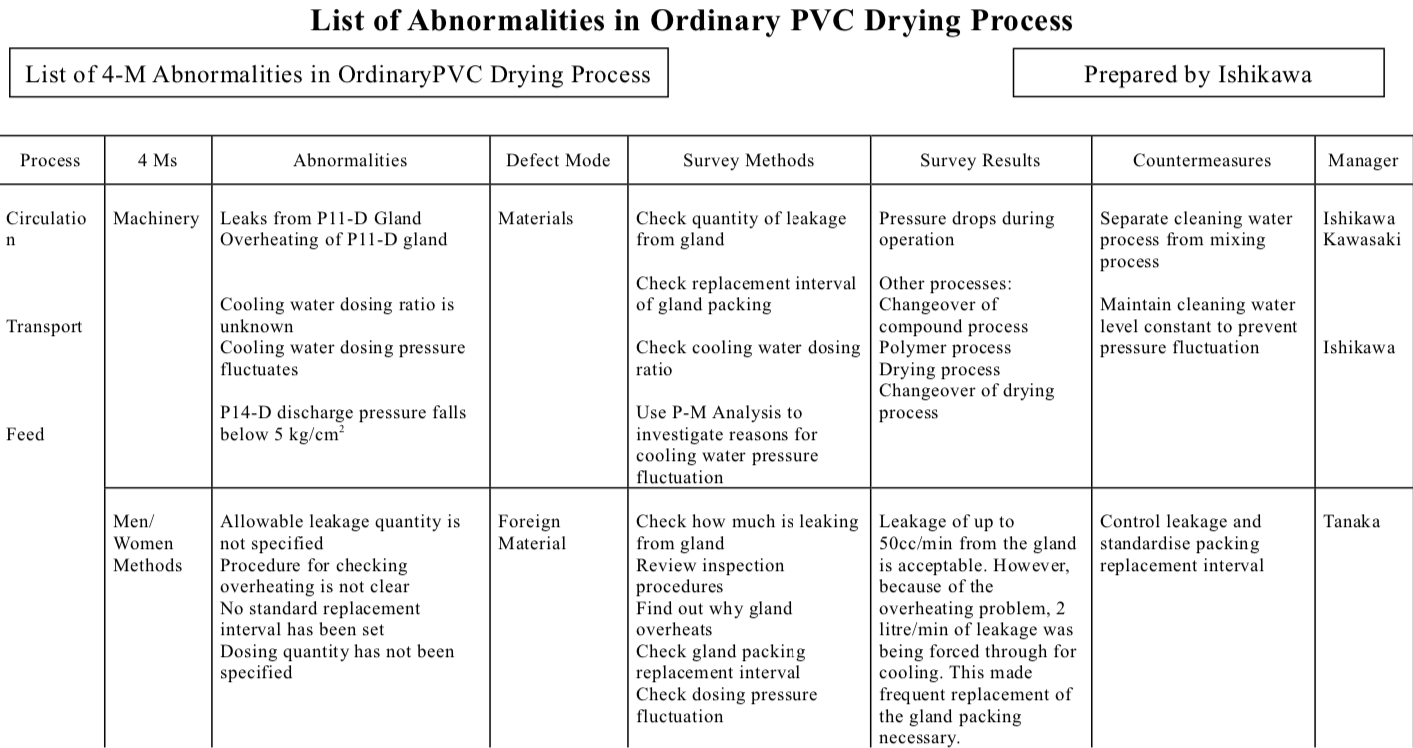
Chart of Countermeasures for Deficiencies in the 4 Ms