In quality management and food safety, three key concepts are vital in ensuring that products and processes meet specified requirements: Validation, Monitoring, and Verification. Although these terms are often used interchangeably, they refer to distinct activities within the quality management process. This document aims to clearly compare these concepts by defining each term, discussing their respective purposes, and offering real-life examples.
Validation
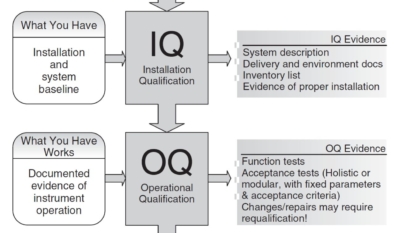
Definition: Validation is obtaining evidence that a control measure or a combination of control measures can effectively control a significant food safety hazard.
Purpose: The primary goal of validation is to ensure that the implemented control measures can deliver the intended results. Validation is applied before an activity, when a control measure is designed, or when changes are made to the implemented control measures.
Example: In a heat treatment process, validation may involve conducting a challenge test to determine the time and temperature combination that effectively eliminates or controls a specific hazard, such as a pathogenic microorganism. By validating the heat treatment process, the food manufacturer can have confidence that the chosen parameters will effectively control the identified hazard.
Monitoring
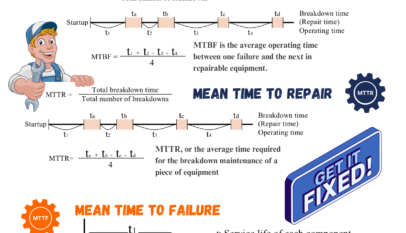
Definition: Monitoring is determining the status of a system, process, or activity by conducting a planned sequence of observations or measurements to assess whether a process is operating as intended.
Purpose: The primary goal of monitoring is to provide information for action within a specified time frame. Monitoring is applied during an activity, enabling timely detection of deviations from the established critical limits and allowing corrective actions to be taken.
Example: During the heat treatment process in food production, continuous monitoring of time and temperature parameters is necessary to ensure that the process remains within the validated parameters. Monitoring can be performed using temperature probes or data loggers, which record temperature data throughout the process.
Verification
Definition: Verification is the confirmation, through the provision of objective evidence, that specified requirements have been fulfilled.
Purpose: The primary goal of verification is to provide information to confirm conformity. Verification is applied after activity and is performed periodically to confirm that the process continues to be as effective as when it was first validated. It also ensures that the implemented control measures are functioning as intended.
Example: To verify the effectiveness of the heat treatment process, laboratory analysis of products may be conducted, such as testing for the presence of pathogenic microorganisms or measuring the product’s water activity. This analysis provides objective evidence that the heat treatment process has successfully controlled the identified hazard.
Conclusion
Validation, Monitoring, and Verification each play unique and critical roles in ensuring food safety and quality management. Validation provides evidence of the capability of control measures to deliver intended results, while monitoring ensures the continuous assessment of processes and enables timely corrective actions. Verification confirms that the implemented control measures are functioning as intended and continue to be effective. Organizations can maintain high-quality processes and products and safeguard public health by understanding the distinctions between these three concepts and systematically implementing them.