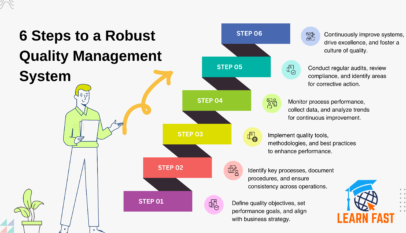
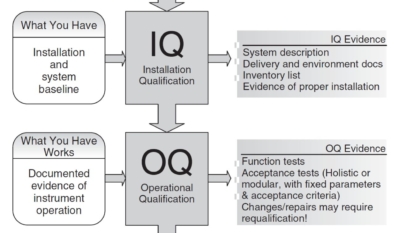
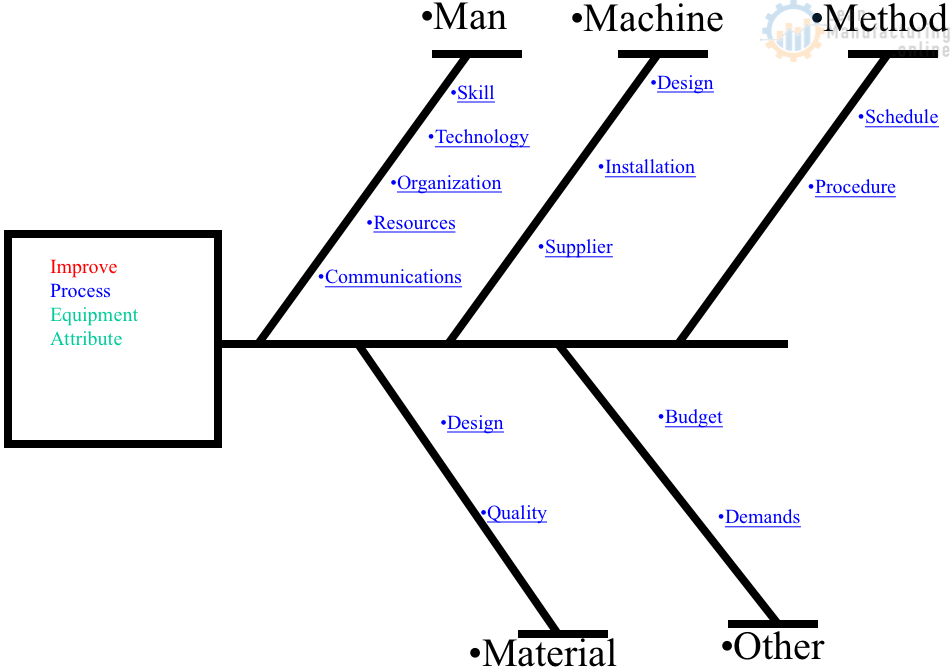
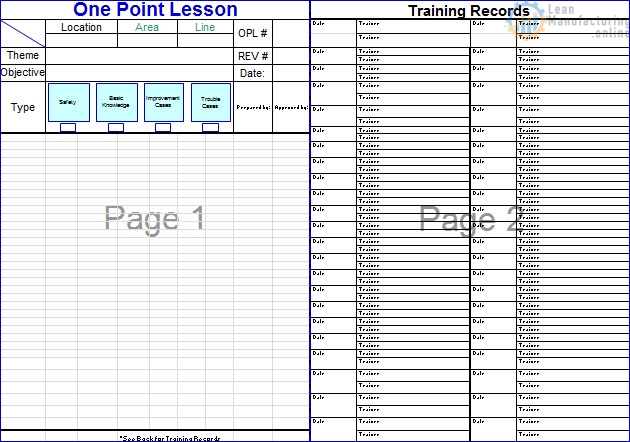
Developing Quality Maintenance. Step 1
Step 2 Investigate the Processes where Defects Occur
Step 3 Investigate and Analyze 4-M Conditions
Step 4 Plan Action to Correct Deficiencies
Step 5 Analyze Situations where the Conditions for Building in Quality Are Unclear
Step 6 and 7 Eliminate Flaws in 4-M Conditions and Finalize
Step 8 Consolidate Checking Methods
Step 9 and 10 Determine Standard Values for Checks and Revise Standards
January 31, 2026
0 1,775
Six Sigma vs Lean Six Sigma: A Practical Guide
December 27, 2025
0 2,404
How to Improve Manufacturing Processes with the RIVET Method
Artificial Intelligence
Continuous Improvement
Lean
Lean Manufacturing
Lean Six Sigma
Productivity
Safety
TPM
World Class Manufacturing

November 19, 2025
0 2,538
Gemba Walk GPT: The Smartest Way to Turn Observations Into Real Improvement
November 16, 2025
0 2,907
How to Create a Step-by-Step Reveal Animation in PowerPoint
Book reviews
Continuous Improvement
Leadership and Continuous Improvement
Lean Manufacturing
Lean Six Sigma

October 4, 2025
0 3,969
Explore the Best Free Online Libraries in 2025
October 3, 2025
0 3,843
TPM Master Plan: The Roadmap to World-Class Manufacturing
September 19, 2025
0 1,277
Mastering Acceptance Quality Level (AQL): A Practical Guide
August 6, 2025
0 1,467
The Complete Guide to GD&T: Symbols, Rules, and Best Practices
June 14, 2024
0 6,066
Essential Quality Tools for Effective Process Improvement
November 9, 2023
0 2,057
Unlocking the Secrets of Pharmaceutical Validation: A Comprehensive Training Guide
October 6, 2023
0 1,544
What is the purpose of auditing?
October 2, 2023
0 1,678
The Cornerstones of Good Manufacturing Practice: A Deep Dive
Most Popular
4M Analysis Process
The purpose of this procedure is to define the steps to do a 4M …