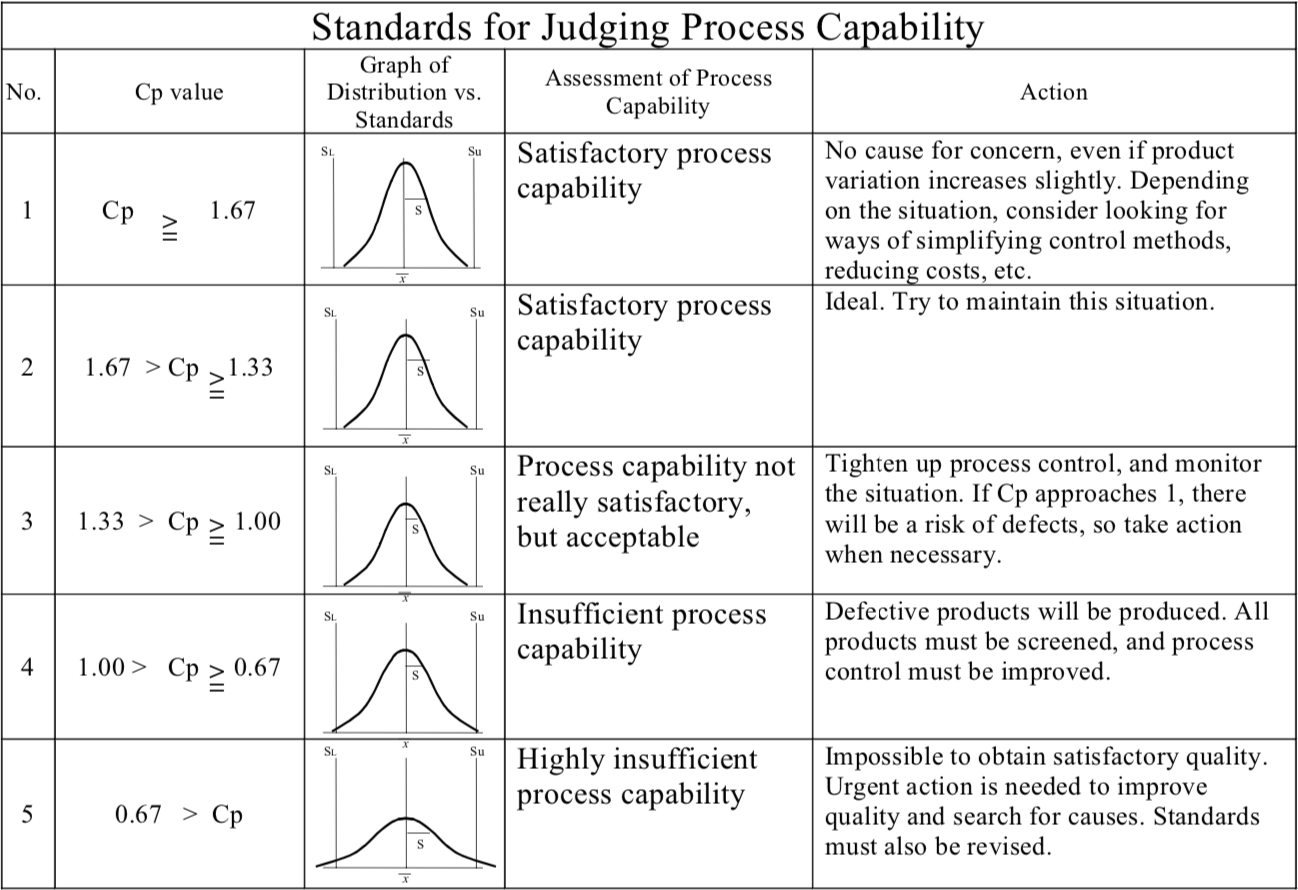
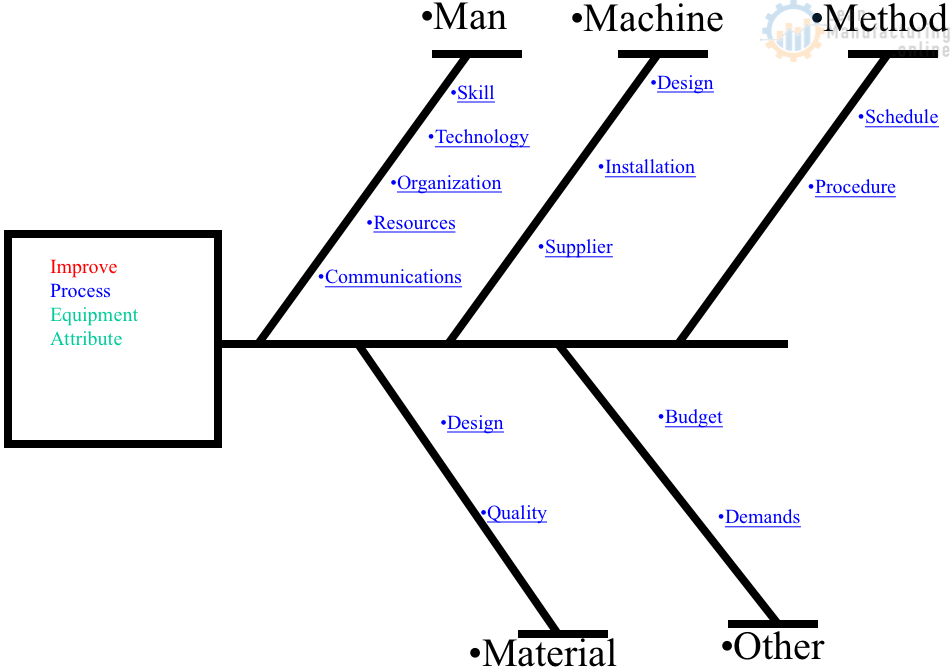
Raising the Cp value (see Table “Assessing Quality Levels”) means minimizing any variations in the quality characteristics.
A practical approach for eliminating variations is based on eradicating minor imperfections in the machinery, jigs and tools, measuring equipment, materials, and work environment.
Calculating the process capability index
If a standard has upper and lower limits, the process capability index, Cp (or Cpk), is given by the formula:

Where the process mean does not coincide with the mean value of the product’s quality specification, a correction factor k must be applied: